Abstract
A fraction of lower risk MDS patients benefit from approved therapies but generally for a limited duration, and several require experimental therapies.1 Rigosertib, a small molecule inhibitor of RAS effector pathways, has been tested in phase I and II trails at our center. We present case histories of two patients with lower-risk MDS who experienced a complete response to Rigosertib which persisted for more than five years.
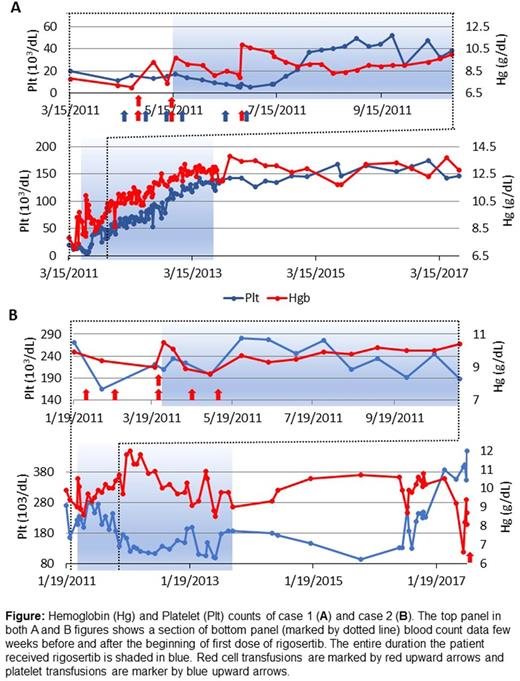
Case 1: A 63-year-old Hispanic female with history of aplastic anemia and PNH since 1990 was diagnosed with low grade MDS in 2011. She was previously treated with mycophenolate mofetil, cyclosporine, and prednisone, which improved her counts. Subsequently, the counts deteriorated and she became heavily transfusion dependent for both red cells and platelets; WBC 1.5 × 103/μL, hemoglobin 7.2 g/dL, and platelet count 11,000/μL. A bone marrow biopsy in 4/11 showed a hypo- to normocellular marrow, subtle dysplastic changes. In August 2011, she started a phase 1 clinical trial of Rigosertib (ON01910.Na) taking 570mg twice a day for 14 of 21-day cycles. She responded well to rigosertib, and within three months, she stopped requiring blood and platelet transfusions (Fig 1A). After 18 months of treatment, she came off the study and has continued to have normal blood counts since. The latest CBC on July 10, 2017 showed WBC 3.8, Hb 12.8 and platelets 146,000. BM from the same day showed a mildly hypocellular (20-30%) marrow with patchy trilineage hematopoiesis and relative expansion of erythroid colonies. Myeloid elements and megakaryocytes were reduced. Megakaryocytes were not dysplastic. Cytogenetics were normal. She enjoys a normal quality of life.
Case 2: A 78-year-old white male was diagnosed with refractory cytopenia with multilineage dysplasia in January 2009. He had low blood cell counts five years prior to diagnosis, becoming progressively worse over time. A BM showed multi-lineage dysplasia, no increase in blasts and normal cytogenetics. He initially had a good response to 40,000 and then to 60,000 unit erythropoietin (EPO) per week for about a year but became transfusion dependent. In 2010, he started Revlimid with an initial response but treatment was discontinued due to severe rash. In March 2011, he began the same phase 1 clinical trial as patient 1. He was instructed to take investigational drug Rigosertib (ON01910.Na) 280mg caps (60 tablets) 2 caps along with 70 mg caps (60 tablets) twice a day for 14 days of the 21-day cycle. He also started darbepoeitin 500mg every two weeks. Within 8 weeks of starting Rigosertib, he became transfusion independent and remained so for almost six years (Fig 1B). Because of periodic episodes of dysuria he discontinued rigosertib in October 2013 but continued darbepoetin intermittently. Gene sequencing in May 2014 showed pathogenic somatic mutations in ASXL1 and U2AF1 and mutations of unknown significance in SRSF2 and PHF6 . genes. The patient remained transfusion independent until June 2017 when he required a transfusion. He received darbepoetin 500ug on 4/4, 4/18, 5/2, 5/16, 5/30, 6/13, and 7/5/17. In the last 8 weeks, he has required multiple transfusions. While it is possible that the long term response in this case is related to Epo rather than Rigosertib, the patient had clearly become transfusion dependent after losing response to Epo. He only regained transfusion independence when rigosertib was added to the regimen.
Conclusion: The precise mechanism of action of Rigosertib in lower risk MDS remains unknown, but it has been proposed that the overall biological effects appear to be mediated by direct binding of the compound to the Ras-binding domain (RBD) found in many Ras effector proteins involved in signal transduction, including Raf kinases and PI3K. Upon treatment with Rigosertib, cells either undergo apoptosis or differentiation, thereby potentially reducing the clone size and producing a clinical response. Despite known point mutations in RAS in solid and liquid malignancies, it has been a difficult target. The allosteric mechanism by which Rigosertib blocks signaling proteins downstream of RAS represents an exciting novel approach for making this oncogene into a "druggable" target. The depth of responses in these two cases as well as the duration, suggest that Rigosertib exerts its effects at a stem cell level
Raza: Janssen R&D: Research Funding; Celgene Inc.: Research Funding; Genoptix: Speakers Bureau; Novartis: Speakers Bureau; Kura Oncology: Research Funding; Onconova Therapeutics: Research Funding, Speakers Bureau; Syros Pharmaceuticals: Research Funding. Heaney: Onconova Therapeutics: Research Funding; Novartis: Consultancy. Ali: Onconova Therapeutics: Consultancy; Kura Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.